Human papillomavirus
| Human papillomavirus | |
|---|---|
_EM.jpg) |
|
| TEM of papillomavirus | |
| Virus classification | |
| Group: | Group I (dsDNA) |
| Order: | Unranked |
| Family: | Papillomaviridae |
| Genera | |
|
Alphapapillomavirus |
|
| Human Papilloma Viruses | |
|---|---|
| Classification and external resources | |
| ICD-10 | B97.7 |
| ICD-9 | 078.1 079.4 |
| DiseasesDB | 6032 |
| eMedicine | med/1037 |
| MeSH | D030361 |
A human papillomavirus (HPV) is a member of the papillomavirus family of viruses that is capable of infecting humans. Like all papillomaviruses, HPVs establish productive infections only in the stratified epithelium of the skin or mucous membranes. While the majority of the nearly 200 known types of HPV cause no symptoms in most people, some types can cause warts (verrucae), while others can – in a minority of cases – lead to cancers of the cervix, vulva, vagina, and anus in women or cancers of the anus and penis in men.[1]
More than 30 to 40 types of HPV are typically transmitted through sexual contact and infect the anogenital region. Some sexually transmitted HPV types may cause genital warts. Persistent infection with "high-risk" HPV types—different from the ones that cause skin warts—may progress to precancerous lesions and invasive cancer.[2] HPV infection is a cause of nearly all cases of cervical cancer;[3] however, most infections with these types do not cause disease.
Most HPV infections in young females are temporary and have little long-term significance. 70% of infections are gone in 1 year and 90% in 2 years.[4] However, when infection persists—in 5% to 10% of infected women—there is high risk of developing cervical precancer (lesions on the cervix), which can progress to invasive cervical cancer. This process usually takes 15–20 years, providing many opportunities for detection and treatment of the pre-cancerous condition, often with high cure rates.
In the US and other high-resource countries, a cervical Papanicolaou (Pap) test is used to detect abnormal cells which may develop into cancer. A cervical examination also detects warts and other abnormal growths which become visible as white patches of skin after they are washed with acetic acid (visual inspection). Abnormal and cancerous areas can be removed with a simple procedure, typically with a cauterizing loop or—more common in the developing world—by freezing (cryotherapy). New HPV DNA tests are more sensitive than Pap or visual inspection and a lower-cost HPV test suitable for low-resource settings may become available soon, potentially making high-sensitivity screening feasible where it currently does not exist in Africa, Asia and Latin America.
Pap smears have reduced the incidence and fatalities of cervical cancer in the developed world, but even so there were 11,000 cases and 3,900 deaths in the U.S. in 2008. Cervical cancer has substantial mortality in resource-poor areas; worldwide, there are 490,000 cases and 270,000 deaths.[5][6] In large part because Pap is difficult to sustain in low-resource settings, eighty to 85 percent of cervical cancer deaths occur in the developing world.
HPV vaccines, Cervarix and Gardasil, which prevent infection with the HPV types (16 and 18) that cause 70% of cervical cancer, may lead to further decreases.[5][7]
Contents |
History
In 1976 Harald zur Hausen published the hypothesis that human papilloma virus plays an important role in the cause of cervical cancer. In 1983 and 1984 zur Hausen and his collaborators identified HPV16 and HPV18 in cervical cancer.[8] In 1972, the association of the Human papilloma viruses with skin cancer in epidermodysplasia verruciformis was proposed by Stefania Jablonska in Poland. In 1978 Jablonska and Gerard Orth at the Pasteur Institute discovered HPV-5 in skin cancer.[9]
Prevalence
United States of America
HPV is estimated to be the most common sexually transmitted infection in the United States.[10] Most sexually active men and women will probably acquire genital HPV infection at some point in their lives.[11] The American Social Health Association reported estimates that about 75-80% of sexually active Americans will be infected with HPV at some point in their lifetime.[12][13] By the age of 50 more than 80% of American women will have contracted at least one strain of genital HPV.[14][15][16]
It was estimated that in the year 2000, there were approximately 6.2 million new HPV infections among Americans aged 15–44; of these, an estimated 74% occurred to people between ages 15–24.[17] Of the STDs studied, genital HPV was the most commonly acquired.[17]
Estimates of HPV prevalence vary from 14% to more than 90%.[18] One reason for the difference is that some studies report women who currently have a detectable infection, while other studies report women who have ever had a detectable infection.[19][20] Another cause of discrepancy is the difference in strains that were tested for.
One study found that, during 2003–2004, at any given time, 26.8% of women aged 14 to 59 were infected with at least one type of HPV. This was higher than previous estimates. 15.2% were infected with one or more of the high-risk types that can cause cancer. However only 3.4% were infected with one or more of the four types prevented by the Gardasil vaccine, which was lower than previous estimates.[10][21]
| Age (years) | Prevalence (%) |
|---|---|
| 14 to 19 | 24.5% |
| 20 to 24 | 44.8% |
| 25 to 29 | 27.4% |
| 30 to 39 | 27.5% |
| 40 to 49 | 25.2% |
| 50 to 59 | 19.6% |
| 14 to 59 | 26.8% |
The prevalence for high-risk and low-risk types is roughly similar over time.[22] The overall prevalence of high- and low-risk HPV types was 15.2% and 17.8%, respectively.
Note that prevalence decreases with age. This may be due to HPV infection being cleared by the immune system, or sinking to undetectable levels while still present in the body. HPV will probably remain in the infected person's cells for an indefinite time—most often in a latent state, but occasionally producing symptoms or disease. Recent studies from the Albert Einstein College of Medicine and from the University of Washington suggest that HPV may eventually be cleared in most people with well functioning immune systems. It appears that in some cases the virus does remain in the body indefinitely, producing symptoms if the immune system weakens.
Cervical cancer
The American Cancer Society estimates that in 2008, about 11,070 women in the United States will be diagnosed with invasive cervical cancer, and about 3,870 US women will die from this disease.[23]
Life cycle
HPV infection is limited to the basal cells of stratified epithelium, the only tissue in which they replicate.[24] The virus can not bind to live tissue; instead it infects epithelial tissues through micro-abrasions or other epithelial trauma that exposes segments of the basement membrane.[24] The infectious process is slow, taking 12–24 h for initiation of transcription. It's believed that involved antibodies play a major neutralizing role while the virions still reside on the basement membrane and cell surfaces.[24]
E6/E7 proteins
E6 and E7 are the HPV proteins associated with cancer.
- E6/E7 in the HPV life cycle
The HPV genome is composed of six early (E1, E2, E3, E4, E6 and E7) and two late (L1 and L2) proteins.[25] After the host cell is infected E1 and E2 are expressed first. High E2 levels represses E6/E7. When the host and HPV genomes integrate E2 function is disrupted as well E6/E7 repression.
- E6/E7 in cancer
The E6/E7 proteins inactivate two tumor suppressor protein, p53 (E6) and pRb (E7).[26] The viral oncogenes, E6 and E7[27] are thought to modify the cell cycle so as to retain the differentiating host keratinocyte in a state that is favourable to the amplification of viral genome replication and consequent late gene expression. E6 in association with host E6 associated protein, which has ubiquitin ligase activity, act to ubiquitinate p53, leading to its proteosomal degradation. E7 (in oncogenic HPVs) acts as the primary transforming protein. E7 competes for retinoblastoma protein (pRb) binding, freeing the transcription factor E2F to transactivate its targets, thus pushing the cell cycle forwards. All HPV can induce transient proliferation, but only 16 and 18 can immortalise cell intes (in vitro). It has also been shown that HPV 16 and 18 cannot immortalise primary rat cells alone; there needs to be activation of the ras oncogene. In the upper layers of the host epithelium, the late genes L1 and L2 are transcribed/translated and serve as structural proteins which encapsidate (Encapsidation is the process of incorporating a nucleic acid sequence (e.g., a vector, or a viral genome) into a viral particle) the amplified viral genomes. Once the genome is encapsidated, the capsid appears to undergo a redox-dependent assembly/maturation event which is tied to a natural redox gradient that spans both suprabasal and cornified epithelial tissue layers. This assembly/maturation event stabilizes virions, and increases their specific infectivity.[28] Virions can then be sloughed off in the dead squames of the host epithelium and the viral lifecycle continues.[29] A 2010 study has found that E6 and E7 are involved in beta-catenin nuclear accumulation and activation of Wnt signaling in HPV-induced cancers.[30]
Latency period
Once an HPV viron invades a cell, an active infection occurs, and the virus can be transmitted. Several months to years may elapse before squamous intraepithelial lesions (SIL) develop and can be clinically detected. The time from active infection to clinically detectable disease makes it difficult for someone who has become infected to establish which partner was the source of infection.
Types and associated diseases

Over 120 HPV types have been identified and are referred to by number.[26] Types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 are "high-risk" sexually transmitted HPVs and may lead to the development of cervical intraepithelial neoplasia (CIN), vulvar intraepithelial neoplasia (VIN), penile intraepithelial neoplasia (PIN), and/or anal intraepithelial neoplasia (AIN).
| Disease | HPV type |
|---|---|
| Common warts | 2, 7 |
| Plantar warts | 1, 2, 4, 63 |
| Flat warts | 3, 10 |
| Anogenital warts | 6, 11, 42, 44 and others[31] |
| Genital cancers | |
| Epidermodysplasia verruciformis | more than 15 types |
| Focal epithelial hyperplasia (oral) | 13, 32 |
| Oral papillomas | 6, 7, 11, 16, 32 |
| Oropharyngeal cancer | 16 |
| Laryngeal papillomatosis | 6,11 |
Cancers

About a dozen HPV types (including types 16, 18, 31 and 45) are called "high-risk" types because they can lead to cervical cancer, as well as anal cancer, vulvar cancer and penile cancer[33] . Several types of HPV, particularly type 16, have been found to be associated with oropharyngeal squamous-cell carcinoma, a form of head and neck cancer.[34][35] HPV-induced cancers often have viral sequences integrated into the cellular DNA. Some of the HPV "early" genes, such as E6 and E7, are known to act as oncogenes that promote tumor growth and malignant transformation. Oral infection with HPV increased the risk of oropharyngeal cancer independent of tobacco and alcohol use.[35]
The E6 and E7 proteins work by inhibiting tumor suppression genes involved in the p53 and the retinoblastoma protein (RB) metabolic pathways: E6 inhibits p53, while E7 inhibits p53, p21 and RB.
The p53 protein prevents cell growth and stimulates apoptosis in the presence of DNA damage. It causes BAX protein upregulation, which blocks the anti-apoptotic effects of the mitochondrial BCL-2 protein. In addition, p53 also upregulates the p21 protein, which blocks the formation of the Cyclin D/Cdk4 complex, thereby preventing the phosphorylation of RB and, in turn, halting cell cycle progression by preventing the activation of E2F. In short, p53 is a tumor suppressor gene that arrests the cell cycle when there is DNA damage.
E6 has a close relationship with the cellular protein E6-AP (E6-associated protein). E6-AP is involved in the ubiquitin ligase pathway. A system which acts to degrade proteins. E6-AP binds ubiquitin to the p53 protein, thereby flagging it for proteosomal degradation.

An infection with one or more high-risk HPV types is believed to be a prerequisite for the development of cervical cancer (the vast majority of HPV infections are not high risk); according to the American Cancer Society, women with no history of the virus do not develop this type of cancer. However, most HPV infections are cleared rapidly by the immune system and do not progress to cervical cancer. Because the process of transforming normal cervical cells into cancerous ones is slow, cancer occurs in people who have been infected with HPV for a long time, usually over a decade or more (persistent infection).[36][37]
Sexually-transmitted HPVs also cause a major fraction of anal cancers and approximately 25% of cancers of the mouth and upper throat (the oropharynx) (see figure).[33] The latter commonly present in the tonsil area, and HPV is linked to the increase in oral cancers in non-smokers.[38][39] Engaging in anal sex or oral sex with an HPV-infected partner may increase the risk of developing these types of cancers.[34]
Studies show a link between HPV infection and penile and anal cancer,[33] and the risk for anal cancer is 17 to 31 times higher among gay and bisexual men than among heterosexual men.[40][41] It has been suggested that anal Pap smear screening for anal cancer might benefit some sub-populations of men or women who engage in anal sex.[42] There is no consensus that such screening is beneficial, or who should get an anal Pap smear.[43][44]
Further studies have also shown a link between a wide range of HPV types and squamous cell carcinoma of the skin. In vitro studies suggest that the E6 protein of the HPV types implicated may inhibit apoptosis induced by ultraviolet light.[45]
Warts
Skin warts
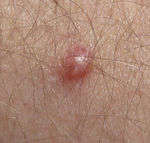
Some HPV infections can cause warts (verrucae), which are noncancerous skin growths. Infection with these types of HPV causes a rapid growth of cells on the outer layer of the skin.[46] Types of warts include:
- Common warts: Some "cutaneous" HPV types, such as HPV-1 and HPV-2, cause common skin warts. Common warts are often found on the hands and feet, but can also occur in other areas, such as the elbows or knees. Common warts have a characteristic cauliflower-like surface and are typically slightly raised above the surrounding skin. Cutaneous HPV types can cause genital warts but are not associated with the development of cancer.
- Plantar warts are found on the soles of the feet. Plantar warts grow inward, generally causing pain when walking.
- Subungual or periungual warts form under the fingernail (subungual), around the fingernail or on the cuticle (periungual). They may be more difficult to treat than warts in other locations.[47]
- Flat warts: Flat warts are most commonly found on the arms, face or forehead. Like common warts, flat warts occur most frequently in children and teens. In people with normal immune function, flat warts are not associated with the development of cancer.[48]
Genital warts are quite contagious, while common, flat, and plantar warts are much less likely to spread from person to person.
Genital warts
Genital or anal warts (condylomata acuminata or venereal warts) are the most easily recognized sign of genital HPV infection. Although a wide variety of HPV types can cause genital warts, types 6 and 11 account for about 90% of all cases.[49][50]
Most people who acquire genital wart-associated HPV types clear the infection rapidly without ever developing warts or any other symptoms. People may transmit the virus to others even if they do not display overt symptoms of infection.
HPV types that tend to cause genital warts are not those that cause cervical cancer.[1] However, since an individual can be infected with multiple types of HPV, the presence of warts does not rule out the possibility of high-risk types of the virus also being present.
The types of HPV that cause genital warts are usually different from the types that cause warts on other parts of the body, such as the hands or inner thighs.
Respiratory papillomatosis
HPV types 6 and 11 can cause a rare condition known as recurrent respiratory papillomatosis, in which warts form on the larynx[51] or other areas of the respiratory tract.[37][52]
These warts can recur frequently, may require repetitive surgery, may interfere with breathing, and in extremely rare cases can progress to cancer.[37][53]
HPV in immunocompromised patients
In very rare cases, HPV may cause epidermodysplasia verruciformis in immunocompromised individuals. The virus, unchecked by the immune system, causes the overproduction of keratin by skin cells, resulting in lesions resembling warts or cutaneous horns.[54]
For instance, Dede Koswara, an Indonesian man developed warts that spread across his body and became root-like growths. Attempted treatment by both Indonesian and American doctors included surgical removal of the warts.
Infection prevention
Condoms offer some protection against genital infection,[55] but any exposed skin can transmit the virus. Genital HPV infection is the most frequent sexually transmitted disease in the world.[56]
Vaccines
Two vaccines are available to prevent infection by some HPV types, Gardasil, marketed by Merck and Cervarix, marketed by GlaxoSmithKline. Both protect against initial infection with HPV types 16 and 18, which cause most of the HPV associated cancer cases. Gardasil also protects against HPV types 6 and 11, which cause 90% of genital warts.
The vaccines provide little benefit to women who have already been infected with HPV types 16 and 18—which includes most sexually active females. For this reason the vaccine is recommended primarily for those women who have not yet been exposed to HPV during sex. The World Health Organization position paper on HPV vaccination clearly outlines appropriate, cost-effective strategies for using HPV vaccine in public sector programs.[57]
Both are delivered in three shots over six months. In most countries they are approved only for female use, but are approved for male use in relevant countries like USA and UK. The vaccine has not any therapeutic effect on existing HPV infection or cervical lesions.[58]
Women should continue to seek cervical screening, such as Pap smear testing, even after receiving the vaccine. Cervical cancer screening recommendations have not changed for females who receive HPV vaccine.[58] Without continued screening, the number of cervical cancers preventable by vaccination alone is less than the number of cervical cancers prevented by regular screening alone.[59][60]
Both men and women are carriers of HPV.[61] Possible benefits and efficacy of vaccinating men are being studied. According to a study by Harvard University Medical School, to vaccinate boys may not be cost effective, especially if a widespread vaccination of girls continues.
No efficacy trials for children under 15 have been performed.[59] Duration of vaccine efficacy is not yet answered by rigorous methodologic trials. Cervarix efficacy is proven for 7.4 years with published data through 6.4 years while Gardasil efficacy is proven for 5 years.[59] Age of vaccination is less important than the duration of efficacy.[59]
Microbicides
Ongoing research has suggested that several inexpensive chemicals might serve to block HPV transmission if applied to the genitals prior to sexual contact.[62] These candidate agents, known as topical microbicides, are currently undergoing clinical efficacy testing. A recent study indicates that some sexual lubricant brands that use a gelling agent called carrageenan prevent papillomavirus infection in animal model systems. [63][64] Clinical trial results announced at the 2010 International Papillomavirus Conference indicate that a carrageenan-based personal lubricant called Carraguard is effective for preventing HPV infection in women.[65] The results suggest that use of carrageenan-based personal lubricant products, such as Divine No 9, Bioglide and Oceanus Carrageenan may likewise be effective for preventing HPV infection.
Cervical and female genital infection
Since cervical and female genital infection by specific HPV types (see above) is highly associated with cervical cancer, those types of HPV infection have received most of the attention from scientific studies.
HPV infections in that area are transmitted primarily via sexual activity.[66]
At least 40 identified HPV types infect the genital tract. If a college woman has at least one different partner per year for four years, the probability that she will leave college with an HPV infection is greater than 85%.[67] Condoms do not protect from the virus because the areas around the genitals including the inner thigh area are not covered, thus exposing these areas to the infected person’s skin.[67]
Condoms
The Centers for Disease Control and Prevention says that male "condom use may reduce the risk for genital human papillomavirus (HPV) infection" but provide a lesser degree of protection compared with other sexual transmitted diseases "because HPV also may be transmitted by exposure to areas (e.g., infected skin or mucosal surfaces) that are not covered or protected by the condom."[68]
Studies have suggested that regular condom use can effectively limit the ongoing persistence and spread of HPV to additional genital sites in individuals who are already infected.[69][70] Thus, condom use reduces the risk that already infected individuals will progress to cervical cancer or develop genital warts.[55]
Smoking avoidance
Tobacco smokers are less likely to develop HPV antibodies.[26]
Sharing of possible contaminated objects should be avoided.[71][72][73] Although possible, transmission by routes other than sexual intercourse is less common for female genital HPV infection.[66] Fingers-genital contact is a possible way of transmission but unlikely to be a significant source.[74][75]
Oral infection
A review of scientific studies in healthy subjects has found carcinogenic HPV in 3.5% of the studies subjects and HPV16 in 1.3%.[76] Men have higher prevalence of oral HPV than women.[26]
Oral HPV infection is associated with HPV-positive oropharyngeal cancer. Odds of oral HPV infection increases with the number of recent oral sex partners or open-mouthed kissing partners.[77] Nonsexual oral infection through salivary or cross transmission is also plausible.[26][78]
Testing for infection
Cervical infection
In March 2003, the U.S. Food and Drug Administration (FDA) approved a test manufactured by Qiagen,[79] which is a "hybrid-capture" test ,[80][81] as the primary screening tool for detecting HPV cervical infection as an adjunct to Pap testing, and may be performed during a routine Pap smear. It can detect the DNA of the 18 HPV types that most commonly affect the cervix and distinguish between "low" and "high-risk" HPV types, but it cannot determine the specific HPV types.[1][82][83]
According to the National Cancer Institute, "testing samples of cervical cells is an effective way to identify high-risk types of HPV that may be present. The FDA has approved an HPV test as a follow-up for women who have an ambiguous Pap test (a screening test to detect cervical cell changes) and, for women over the age of 30, for general cervical cancer screening. This HPV test can identify at least 13 of the high-risk types of HPV associated with the development of cervical cancer. The test can detect high-risk types of HPV even before there are any conclusive visible changes to the cervical cells." [84]
The recent outcomes in the identification of molecular pathways involved in cervical cancer provide helpful information about novel bio- or oncogenic markers that allow monitoring of these essential molecular events in cytological smears, histological or cytological specimens. These bio- or onco- markers are likely to improve the detection of lesions that have a high risk of progression in both primary screening and triage settings. E6 and E7 mRNA detection PreTect HPV-Proofer, (HPV OncoTect) or p16 cell-cycle protein levels are examples of these new molecular markers. According to published results these markers, which are highly sensitive and specific, allow to identify cells going through malignant transformation.[85][86]
Other testing
Although it is possible to test for HPV DNA in other kind of infections ,[87] there are no FDA-approved tests for general screening in the United States[40] or tests approved by the Canadian government,[88] since the testing is inconclusive and considered medically unnecessary.[89]
Genital warts are the only visible sign of low-risk HPV, and can be identified with a visual check. These visible growths, however, are the result of non-carcinogenic HPV types. 5% acetic acid (vinegar) is used to identify both warts and squamous intraepithelial neoplasia (SIL) lesions with limited success[40] by causing abnormal tissue to appear white, but most doctors have found this technique helpful only in moist areas, such as the female genital tract.[40] At this time, there is no HPV test for males.
Treatment
There is currently no cure or treatment for HPV infection.[1][83][90] However, the viral infection, more often than not, clears by itself.[91] Experts do not agree on whether the virus is completely eliminated or reduced to undetectable levels, and it is difficult to know if one is contagious.[92]
Epidemiology
Cutaneous HPVs
Infection with cutaneous HPVs is ubiquitous.[93] Some HPV types, such as HPV-5, may establish infections that persist for the lifetime of the individual without ever manifesting any clinical symptoms. Like remora suckerfish that hitchhike harmlessly on sharks, these HPV types can be thought of as human commensals. Other cutaneous HPVs, such as HPV types 1 or 2, may cause common warts in some infected individuals. Skin warts are most common in childhood and typically appear and regress spontaneously over the course of weeks to months. About 10% of adults also suffer from recurring skin warts. All HPVs are believed to be capable of establishing long-term "latent" infections in small numbers of stem cells present in the skin. Although these latent infections may never be fully eradicated, immunological control is thought to block the appearance of symptoms such as warts. Immunological control is likely HPV type-specific, meaning that an individual may become immunologically resistant to one HPV type while remaining susceptible to other types.
Genital HPVs
A large increase in the incidence of genital HPV infection occurs at the age when individuals begin to engage in sexual activity. The great majority of genital HPV infections never cause any overt symptoms and are cleared by the immune system in a matter of months. As with cutaneous HPVs, immunity is believed to be HPV type-specific. Some infected individuals may fail to bring genital HPV infection under immunological control. Lingering infection with high-risk HPV types, such as HPVs 16, 18, 31 and 45, can lead to the development of cervical cancer or other types of cancer.[94] In addition to persistent infection with high-risk HPV types, epidemiological and molecular data suggest that co-factors such as the cigarette smoke carcinogen benzo[a]pyrene (BaP) enhance development of certain HPV-induced cancers.[95][96]
High-risk HPV types 16 and 18 are together responsible for over 65% of cervical cancer cases.[11][97] Type 16 causes 41 to 54% of cervical cancers,[11][98] and accounts for an even greater majority of HPV-induced vaginal/vulvar cancers,[99] penile cancers, anal cancers and head and neck cancers.[100]
Perinatal transmission
Although genital HPV types are sometimes transmitted from mother to child during birth, the appearance of genital HPV-related diseases in newborns is rare. Perinatal transmission of HPV types 6 and 11 can result in the development of juvenile-onset recurrent respiratory papillomatosis (JORRP). JORRP is very rare, with rates of about 2 cases per 100,000 children in the United States.[37] Although JORRP rates are substantially higher if a woman presents with genital warts at the time of giving birth, the risk of JORRP in such cases is still less than 1%.
DNA testing in resource-poor areas
Many resource-poor areas cannot provide regular screening, and must rely on infrequent screening. A study of cervical cancer screening of 131,746 women in rural India found that a single DNA test reduced the number of advanced cervical cancers and deaths over 8 years, while a single acetic acid examination or a single Pap screening did not. However, the DNA test cost US $30–40, which was unaffordable in many regions, it is time-consuming, and requires a sophisticated laboratory infrastructure. A simple, affordable, and accurate test is being evaluated in China and other countries.[101][102][103] The new test may become available on the market in 2010 at significantly lower cost than current tests.
With HPV testing, there was a 50 percent reduction [104][105] in the number of deaths from cervical cancer compared to unscreened women. Compared to other methods, the research showed the HPV testing reported the fewest false negatives.[106]
See also
- Dyskaryosis
References
- ↑ 1.0 1.1 1.2 1.3 "Genital HPV Infection - CDC Fact Sheet". Centers for Disease Control and Prevention (CDC). April 10, 2008. http://www.cdc.gov/std/HPV/STDFact-HPV.htm. Retrieved 13 November 2009.
- ↑ Schiffman M, Castle PE (August 2003). "Human papillomavirus: epidemiology and public health". Archives of Pathology & Laboratory Medicine 127 (8): 930–4. PMID 12873163. http://journals.allenpress.com/jrnlserv/?request=get-abstract&issn=0003-9985&volume=127&page=930. Retrieved 18 May 2009.
- ↑ Walboomers JM, Jacobs MV, Manos MM (1999). "Human papillomavirus is a necessary cause of invasive cervical cancer worldwide". J. Pathol. 189 (1): 12–9. doi:10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. PMID 10451482.
- ↑ Goldstein MA, Goodman A, del Carmen MG, Wilbur DC (March 2009). "Case records of the Massachusetts General Hospital. Case 10-2009. A 23-year-old woman with an abnormal Papanicolaou smear". N. Engl. J. Med. 360 (13): 1337–44. doi:10.1056/NEJMcpc0810837. PMID 19321871. PMID 19321871
- ↑ 5.0 5.1 Kahn JA (July 2009). "HPV vaccination for the prevention of cervical intraepithelial neoplasia". N. Engl. J. Med. 361 (3): 271–8. doi:10.1056/NEJMct0806938. PMID 19605832. PMID 19605832
- ↑ "NCCC National Cervical Cancer Coalition". http://www.nccc-online.org/. Retrieved 1 July 2008.
- ↑ Lowy DR, Schiller JT (2006). "Prophylactic human papillomavirus vaccines". J. Clin. Invest. 116 (5): 1167–73. doi:10.1172/JCI28607. PMID 16670757.
- ↑ "HPV - the Shy Virus". Soundprint.org radio program. 6 December 2008. http://soundprint.org/radio/display_show/ID/774/name/HPV+-+the+Shy+Virus. Retrieved 6 December 2008.
- ↑ Human papillomaviruses World Health Organization, International Agency for Research on Cancer 2007 ISBN 9789283212904
- ↑ 10.0 10.1 10.2 Dunne EF, Unger ER, Sternberg M (2007). "Prevalence of HPV infection among females in the United States". JAMA 297 (8): 813–9. doi:10.1001/jama.297.8.813. PMID 17327523.
- ↑ 11.0 11.1 11.2 Baseman JG, Koutsky LA (2005). "The epidemiology of human papillomavirus infections". J. Clin. Virol. 32 Suppl 1: S16–24. doi:10.1016/j.jcv.2004.12.008. PMID 15753008. *Note: The authors state on page S17 "Overall, these DNA-based studies, combined with measurements of type-specific antibodies against HPV capsid antigens, have shown that most (>50%) sexually active women have been infected by one or more genital HPV types at some point in time."
- ↑ "American Social Health Association - HPV Resource Center". http://www.ashastd.org/hpv/hpv_learn_men.cfm. Retrieved 17 August 2007.
- ↑ "American Social Health Association - National HPV and Cervical Cancer Prevention Resource Center". http://www.ashastd.org/hpv/hpv_learn_patfactsheet.cfm. Retrieved 1 July 2008.
- ↑ Planned Parenthood "In fact, the lifetime risk for contracting HPV is at least 50 percent for all sexually active women and men, and, it is estimated that, by the age of 50, at least 80 percent of women will have acquired sexually transmitted HPV (CDC, 2004; CDC, 2006)."
- ↑ "Medical News Today". Medical News Today. http://www.medicalnewstoday.com/articles/64137.php. Retrieved 2010-08-29.
- ↑ Dunne EF, Unger ER, Sternberg M (February 2007). "Prevalence of HPV infection among females in the United States". JAMA : the journal of the American Medical Association 297 (8): 813–9. doi:10.1001/jama.297.8.813. PMID 17327523.
- ↑ 17.0 17.1 Hillard Weinstock, Stuart Berman and Willard Cates, Jr. (January/February 2004). "Sexually Transmitted Diseases Among American Youth: Incidence and Prevalence Estimates, 2000". Perspectives on Sexual and Reproductive Health 36 (1): 6. doi:10.1363/3600604. PMID 14982671. http://www.guttmacher.org/pubs/journals/3600604.html.
- ↑ Revzina NV, Diclemente RJ (2005). "Prevalence and incidence of human papillomavirus infection in women in the USA: a systematic review". International journal of STD & AIDS 16 (8): 528–37. doi:10.1258/0956462054679214. PMID 16105186."The prevalence of HPV reported in the assessed studies ranged from 14% to more than 90%."
- ↑ McCullough, Marie (28 February 2007). "Cancer-virus strains rarer than first estimated". The Philadelphia Inquirer. http://www.philly.com/mld/inquirer/living/health/16798039.htm. Retrieved 2 March 2007.
- ↑ Brown, David (28 February 2007). "Study finds more women than expected have HPV". San Francisco Chronicle. http://sfgate.com/cgi-bin/article.cgi?f=/c/a/2007/02/28/MNGOCOCAF61.DTL. Retrieved 2 March 2007. (originally published in the Washington Post as "More American Women Have HPV Than Previously Thought")
- ↑ Lindsey Tanner (March 11, 2008). "Study Finds 1 in 4 US Teens Has a STD". Newsvine. Associated Press. http://www.newsvine.com/_news/2008/03/11/1358811-study-finds-1-in-4-us-teens-has-a-std. Retrieved 17 March 2008.
- ↑ "JAMA - Prevalence of HPV Infection Among Females in the United States, February 28, 2007, Dunne et al. 297 (8): 813, Figure OC70010F1". Jama.ama-assn.org. 2007-02-28. http://jama.ama-assn.org/cgi/content/full/297/8/813/JOC70010F1. Retrieved 2010-08-29.
- ↑ "What Are the Key Statistics About Cervical Cancer?". American Cancer Society. October 28, 2009. http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_statistics_for_cervical_cancer_8.asp. Retrieved 2009-12-04.
- ↑ 24.0 24.1 24.2 doi:10.1016/j.ygyno.2010.04.004
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ PMID 19430123 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ 26.0 26.1 26.2 26.3 26.4 Chaturvedi, Anil; Maura L. Gillison (March 4, 2010). "Human Papillomavirus and Head and Neck Cancer". In Andrew F. Olshan. Epidemiology, Pathogenesis, and Prevention of Head and Neck Cancer (1st ed.). New York: Springer. ISBN 978-1-4419-1471-2. http://www.springerlink.com/content/q676132t254373n5/.
- ↑ PMID 12445661 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Conway MJ, Alam S, Ryndock EJ, et al. (October 2009). "Tissue-spanning redox gradient-dependent assembly of native human papillomavirus type 16 virions". Journal of Virology 83 (20): 10515–26. doi:10.1128/JVI.00731-09. PMID 19656879. PMID 19656879
- ↑ Bryan JT, Brown DR (March 2001). "Transmission of human papillomavirus type 11 infection by desquamated cornified cells". Virology 281 (1): 35–42. doi:10.1006/viro.2000.0777. PMID 11222093. PMID 11222093
- ↑ PMID 20215420 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ 31.0 31.1 31.2 Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; Mitchell, Richard (2007). "Chapter 19 The Female Genital System and Breast". Robbins Basic Pathology (8 ed.). Philadelphia: Saunders. ISBN 1-4160-2973-7.
- ↑ 32.0 32.1 Muñoza N, Castellsaguéb X, Berrington de Gonzálezc A, Gissmann L (2006). "Chapter 1: HPV in the etiology of human cancer". Vaccine 24 (3): S1–S10. doi:10.1016/j.vaccine.2006.05.115. PMID 16949995.
- ↑ 33.0 33.1 33.2 33.3 Parkin DM (2006). "The global health burden of infection-associated cancers in the year 2002". Int. J. Cancer 118 (12): 3030–44. doi:10.1002/ijc.21731. PMID 16404738.
- ↑ 34.0 34.1 D'Souza G, Kreimer AR, Viscidi R (2007). "Case-control study of human papillomavirus and oropharyngeal cancer". N. Engl. J. Med. 356 (19): 1944–56. doi:10.1056/NEJMoa065497. PMID 17494927. http://content.nejm.org/cgi/content/full/356/19/1944.
- ↑ 35.0 35.1 Ridge JA, Glisson BS, Lango MN, et al. "Head and Neck Tumors" in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer Management: A Multidisciplinary Approach. 11 ed. 2008.
- ↑ Greenblatt R.J. 2005. Human papillomaviruses: Diseases, diagnosis, and a possible vaccine. Clinical Microbiology Newsletter, 27(18), 139-145. Abstract available.
- ↑ 37.0 37.1 37.2 37.3 Sinal SH, Woods CR (2005). "Human papillomavirus infections of the genital and respiratory tracts in young children". Seminars in pediatric infectious diseases 16 (4): 306–16. doi:10.1053/j.spid.2005.06.010. PMID 16210110.
- ↑ Gillison ML, Koch WM, Capone RB (2000). "Evidence for a causal association between human papillomavirus and a subset of head and neck cancers". J. Natl. Cancer Inst. 92 (9): 709–20. doi:10.1093/jnci/92.9.709. PMID 10793107.
- ↑ Gillison ML (2006). "Human papillomavirus and prognosis of oropharyngeal squamous cell carcinoma: implications for clinical research in head and neck cancers". J. Clin. Oncol. 24 (36): 5623–5. doi:10.1200/JCO.2006.07.1829. PMID 17179099.
- ↑ 40.0 40.1 40.2 40.3 "HPV and Men - CDC Fact Sheet". Centers for Disease Control and Prevention (CDC). April 3, 2008. http://www.cdc.gov/std/hpv/STDFact-HPV-and-men.htm. Retrieved 13 November 2009.
- ↑ Frisch M, Smith E, Grulich A, Johansen C (2003). "Cancer in a population-based cohort of men and women in registered homosexual partnerships". Am. J. Epidemiol. 157 (11): 966–72. doi:10.1093/aje/kwg067. PMID 12777359. http://171.66.121.65/cgi/content/full/157/11/966. "However, the risk for invasive anal squamous carcinoma, which is believed to be caused by certain types of sexually transmitted human papillomaviruses, notably type 16, was significantly 31-fold elevated at a crude incidence of 25.6 per 100,000 person-years.".
- ↑ Chin-Hong PV, Vittinghoff E, Cranston RD (2005). "Age-related prevalence of anal cancer precursors in homosexual men: the EXPLORE study". J. Natl. Cancer Inst. 97 (12): 896–905. doi:10.1093/jnci/dji163. PMID 15956651.
- ↑ "AIDSmeds Web Exclusives : Pap Smears for Anal Cancer? - by David Evans". AIDSmeds.com. http://www.aidsmeds.com/articles/aids_anal_pap_2042_14727.shtml. Retrieved 2010-08-29.
- ↑ Goldie SJ, Kuntz KM, Weinstein MC, et al. Cost-effectiveness of screening for anal squamous intraepithelial lesions and anal cancer in HIV-negative homosexual and bisexual men. Amer J of Med 108: 634-641, 2000.
- ↑ Karagas MR, Waterboer T, Li Z, Nelson HH, Michael KM, Bavinck JNB, Perry AE, Spencer SK, Daling J, Green AC, Pawlita M (2010). "Genus β human papillomaviruses and incidence of basal cell and squamous cell carcinomas of skin: population based case-control study". BMJ 341: 2986. doi:10.1136/bmj.c2986. PMID 20616098. PMC 2900549. http://www.bmj.com/cgi/content/full/341/jul08_1/c2986
- ↑ Mayo Clinic.com, Common warts, http://www.mayoclinic.com/print/common-warts/DS00370/DSECTION=all&METHOD=print
- ↑ Lountzis NI, Rahman O (2008). "Images in clinical medicine. Digital verrucae". N. Engl. J. Med. 359 (2): 177. doi:10.1056/NEJMicm071912. PMID 18614785. http://content.nejm.org/cgi/pmidlookup?view=short&pmid=18614785&promo=ONFLNS19.
- ↑ MedlinePlus, Warts, http://www.nlm.nih.gov/medlineplus/warts.html#cat42 (general reference with links). Also, see
- ↑ Greer CE, Wheeler CM, Ladner MB (1995). "Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts". J. Clin. Microbiol. 33 (8): 2058–63. PMID 7559948.
- ↑ Gearheart PA, Randall TC, Buckley RM Jr (2004). "Human Papillomavirus". eMedicine. http://www.emedicine.com/med/topic1037.htm.
- ↑ "Photos of larynx Papillomas - Voice Medicine, New York". Voicemedicine.com. http://www.voicemedicine.com/papilloma.htm. Retrieved 2010-08-29.
- ↑ Wu R, Sun S, Steinberg BM (2003). "Requirement of STAT3 activation for differentiation of mucosal stratified squamous epithelium". Mol. Med. 9 (3-4): 77–84. doi:10.2119/2003-00001.Wu. PMID 12865943.
- ↑ Moore CE, Wiatrak BJ, McClatchey KD (1999). "High-risk human papillomavirus types and squamous cell carcinoma in patients with respiratory papillomas". Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 120 (5): 698–705. doi:10.1053/hn.1999.v120.a91773. PMID 10229596.
- ↑ Moore, M. "Tree man 'who grew roots' may be cured" (November 21, 2007). The Telegraph.
- ↑ 55.0 55.1 Manhart LE, Koutsky LA. (2002). "Do condoms prevent genital HPV infection, external genital warts, or cervical neoplasia? A meta-analysis.". Sex Transm Dis 29 (11): 725–35. doi:10.1097/00007435-200211000-00018. PMID 12438912.
- ↑ PMID 20189438 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Human papillomavirus vaccines: World Health Organization position paper. Weekly Epidemiological Record. 2009, 84:118-131. Available at: www.who.int/wer/2009/wer8415.pdf
- ↑ 58.0 58.1 "Quadrivalent Human Papillomavirus Vaccine, Recommendations of the Advisory Committee on Immunization Practices" (PDF). CDC, Morbidity and Mortality Weekly Report Recommendations 56 / RR-2: 17, Cervical Cancer Screening Among Vaccinated Females. March 23, 2007. http://www.cdc.gov/mmwr/PDF/rr/rr5602.pdf.
- ↑ 59.0 59.1 59.2 59.3 doi:10.1097/GCO.0b013e328332c910
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ "An Interview with Dr. Diane M. Harper, HPV Expert". 28 December 2010. http://www.huffingtonpost.com/marcia-g-yerman/an-interview-with-dr-dian_b_405472.html. Retrieved 12 January 2010.
- ↑ "HPV Virus: Information About Human Papillomavirus". WebMD. http://www.webmd.com/sexual-conditions/hpv-genital-warts/hpv-virus-information-about-human-papillomavirus.
- ↑ Howett MK, Kuhl JP (2005). "Microbicides for prevention of transmission of sexually transmitted diseases". Curr. Pharm. Des. 11 (29): 3731–46. doi:10.2174/138161205774580633. PMID 16305508.
- ↑ PMID 16839203 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 17603495 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Dianne Marais, Daniel Gawarecki, Naomi Rutenberg, Bruce Allan, Khatija Ahmed, Lydia Altini, Nazira Cassim, Felicity Gopolang, Margaret Hoffman and Anna-Lise Williamson. (2010). "Carraguard, a vaginal microbicide, protects women against HPV infection". 26th International Papillomavirus Conference, Montreal, Canada. http://www.hpvmedia.org/index.php?option=com_hpv&view=presentation&id=1110.
- ↑ 66.0 66.1 PMID 16950018 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ 67.0 67.1 Egendorf, Laura. Sexually Transmitted Diseases (At Issue Series). New York: Greenhaven Press, 2007.
- ↑ "CDC - Condom Effectiveness - Male Latex Condoms and Sexually Transmitted Diseases". Centers for Disease Control and Prevention (CDC). October 22, 2009. http://www.cdc.gov/condomeffectiveness/latex.htm. Retrieved 23 October 2009.
- ↑ Moscicki AB (2005). "Impact of HPV infection in adolescent populations". The Journal of adolescent health : official publication of the Society for Adolescent Medicine 37 (6 Suppl): S3–9. doi:10.1016/j.jadohealth.2005.09.011. PMID 16310138.
- ↑ Bleeker MC, Berkhof J, Hogewoning CJ (2005). "HPV type concordance in sexual couples determines the effect of condoms on regression of flat penile lesions". Br. J. Cancer 92 (8): 1388–92. doi:10.1038/sj.bjc.6602524. PMID 15812547.
- ↑ PMID 8849195 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 8389707 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 2256864 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 10616355 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 20570905 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 20081557 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 19320589 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ doi:10.1111/j.1600-0463.2010.02620.x
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ Digene HPV HC2 DNA Test Quiagen HPV test
- ↑ "Qiagen to Buy Digene, Maker of Tests for Cancer-Causing Virus". The New York Times. June 4, 2007. http://query.nytimes.com/gst/fullpage.html?sec=health&res=9C06E2DF1130F937A35755C0A9619C8B63.
- ↑ "So Close Together for So Long, and Now One". The Washington Post. August 20, 2007. http://www.washingtonpost.com/wp-dyn/content/article/2007/08/19/AR2007081901430.html.
- ↑ "Qiagen Digene HPV HC2 DNA Test - For detection of human papillomavirus infections". .qiagen.com. http://www1.qiagen.com/Products/digeneHPVTesthc2.aspx. Retrieved 2010-08-29.
- ↑ 83.0 83.1 "HPV Vaccine Information For Young Women". Centers for Disease Control and Prevention (CDC). June 26, 2008. http://www.cdc.gov/std/hpv/STDFact-HPV-vaccine-young-women.htm. Retrieved 13 November 2009.
- ↑ ""National Cancer Institute Fact Sheet"". Cancer.gov. http://www.cancer.gov/cancertopics/factsheet/risk/HPV. Retrieved 2010-08-29.
- ↑ Wentzensen N, von Knebel Doeberitz M (2007). [iospress.metapress.com/openurl.asp?genre=article&issn=0278-0240&volume=23&issue=4&spage=315 "Biomarkers in cervical cancer screening"]. Dis. Markers 23 (4): 315–30. PMID 17627065. iospress.metapress.com/openurl.asp?genre=article&issn=0278-0240&volume=23&issue=4&spage=315. Retrieved 13 November 2009.
- ↑ Molden T, Kraus I, Skomedal H, Nordstrøm T, Karlsen F (June 2007). "PreTect HPV-Proofer: real-time detection and typing of E6/E7 mRNA from carcinogenic human papillomaviruses". J. Virol. Methods 142 (1-2): 204–12. doi:10.1016/j.jviromet.2007.01.036. PMID 17379322.
- ↑ Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR (2006). "Prevalence of HPV infection among men: A systematic review of the literature". J. Infect. Dis. 194 (8): 1044–57. doi:10.1086/507432. PMID 16991079.
- ↑ "Human Papillomavirus (HPV) and Men: Questions and Answers". 2007. http://www.phac-aspc.gc.ca/std-mts/hpv-vph/hpv-vph-man-eng.php. Retrieved 10 September 2008. "Currently, in Canada there is an HPV DNA test approved for women but not for men."
- ↑ "What Men Need to Know About HPV". 2006. http://www.thehpvtest.com/HPV-for-men-FAQ.html#testmen. Retrieved 4 April 2007. "There is currently no FDA-approved test to detect HPV in men. That is because an effective, reliable way to collect a sample of male genital skin cells, which would allow detection of HPV, has yet to be developed."
- ↑ American Cancer Society. "What Are the Risk Factors for Cervical Cancer?". http://www.cancer.org/docroot/CRI/content/CRI_2_4_2X_What_are_the_risk_factors_for_cervical_cancer_8.asp. Retrieved 21 February 2008.
- ↑ "Cure for HPV". Webmd.com. http://www.webmd.com/sexual-conditions/hpv-genital-warts/hpv-treatment-is-there-hpv-cure. Retrieved 2010-08-29.
- ↑ Gilbert LK, Alexander L, Grosshans JF, Jolley L (2003). "Answering frequently asked questions about HPV.". Sex Transm Dis 30 (3): 193–4. doi:10.1097/00007435-200303000-00002. PMID 12616133. Free full-text.
- ↑ Antonsson A, Forslund O, Ekberg H, Sterner G, Hansson BG (2000). "The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses". J. Virol. 74 (24): 11636–41. doi:10.1128/JVI.74.24.11636-11641.2000. PMID 11090162.
- ↑ Schiffman M, Castle PE (2005). "The promise of global cervical-cancer prevention". N. Engl. J. Med. 353 (20): 2101–4. doi:10.1056/NEJMp058171. PMID 16291978.
- ↑ Alam S, Conway MJ, Chen HS, Meyers C (2007). "Cigarette Smoke Carcinogen Benzo[apyrene Enhances Human Papillomavirus Synthesis"]. J Virol 82 (2): 1053. doi:10.1128/JVI.01813-07. PMID 17989183.
- ↑ "Epidemiologic Factors Associated with Seropositivity to Human Papillomavirus Type 16 and 18 Virus–Like Particles and Risk of Subsequent Infection in Men — Cancer Epidemiology, Biomarkers & Prevention". Cebp.aacrjournals.org. 2010-01-19. http://cebp.aacrjournals.org/content/early/2010/01/18/1055-9965.EPI-09-0790.abstract. Retrieved 2010-08-29.
- ↑ Cohen J (2005). "Public health. High hopes and dilemmas for a cervical cancer vaccine". Science 308 (5722): 618–21. doi:10.1126/science.308.5722.618. PMID 15860602.
- ↑ Noel J, Lespagnard L, Fayt I, Verhest A, Dargent J (2001). "Evidence of human papilloma virus infection but lack of Epstein-Barr virus in lymphoepithelioma-like carcinoma of uterine cervix: report of two cases and review of the literature". Hum. Pathol. 32 (1): 135–8. doi:10.1053/hupa.2001.20901. PMID 11172309.
- ↑ "Vulvar Intraepithelial Neoplasia: Varied signs, varied symptoms: what you need to know". www.advanceweb.com. http://nurse-practitioners.advanceweb.com/Article/Vulvar-Intraepithelial-Neoplasia.aspx. Retrieved 5 August 2009.
- ↑ Bolt J, Vo QN, Kim WJ, McWhorter AJ, Thomson J, Hagensee ME, Friedlander P, Brown KD, Gilbert J (2005). "The ATM/p53 pathway is commonly targeted for inactivation in squamous cell carcinoma of the head and neck (SCCHN) by multiple molecular mechanisms". Oral Oncol. 41 (10): 1013–20. doi:10.1016/j.oraloncology.2005.06.003. PMID 16139561.
- ↑ "New England Journal of Medicine, HPV Screening for Cervical Cancer in Rural India”". Content.nejm.org. 1970-01-01. http://content.nejm.org/cgi/content/full/360/14/1385. Retrieved 2010-08-29.
- ↑ Emery, Gene. "“Reuters- QIAGEN virus test cuts death from cervical cancer”". Reuters.com. http://www.reuters.com/article/healthNews/idUSTRE5307XP20090401. Retrieved 2010-08-29.
- ↑ DONALD G. McNEIL Jr. (April 6, 2009). "New DNA Test Outperforms the Pap Smear". The New York Times. http://www.nytimes.com/2009/04/07/health/07virus.html.
- ↑ Chase, Marilyn (2009-04-01). "“Bloomberg - Cervical cancer deaths halved by HPV Test, Treatment”". Bloomberg.com. http://www.bloomberg.com/apps/news?pid=20601124&sid=asRUT4ihBQDw. Retrieved 2010-08-29.
- ↑ Sarah Boseley, health editor. "“The Guardian - NHS under pressure for new cervical cancer test provision”". Guardian. http://www.guardian.co.uk/science/2009/apr/02/cervical-cancer-india-test-smear. Retrieved 2010-08-29.
- ↑ Sharples, Tiffany (2009-04-02). "“Time - HPV Test Screens Best for Cervical Cancer”". Time.com. http://www.time.com/time/health/article/0,8599,1889078,00.html. Retrieved 2010-08-29.
External links
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER (2007). "Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP)". MMWR Recomm Rep 56 (RR-2): 1–24. PMID 17380109. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5602a1.htm.
- HPV Fact sheets from the Centers for Disease Control and Prevention
- Myths and misconceptions about HPV – American Social Health Association
- HPV's links to oral cancers peer reviewed information from The Oral Cancer Foundation
- STI: HPV a website information for adolescents
- HPV and pregnancy Answers for those pregnant with HPV
- NOW on PBS: "Vaccine Debate" The political controversy over requiring HPV vaccinations for girls.
- HPV found under the fingernails of young men The Daily, University of Washington
- "Human Papillomavirus (HPV) Vaccines", National Cancer Institute Fact Sheet, US National Institutes of Health, October 22, 2009.
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||